Sulphuric Acid and Sodium Hydroxide
Both acetic and citric acid can be used to neutralize NaOH. Students acquire knowledge about combination reactions.

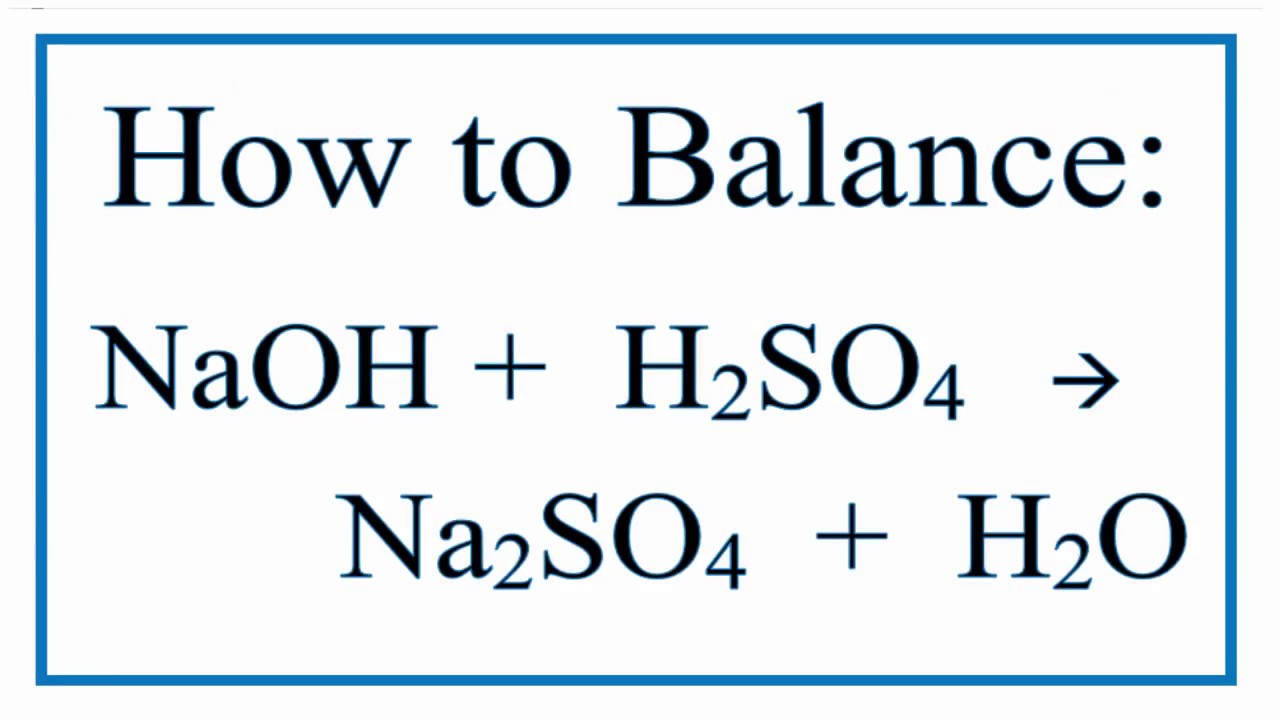
Naoh H2so4 Na2so4 H2o Chemical Equation Balancer
C 19 H 29 COOH.
. Reaction of Acid with Metal Oxides. Ammonium hydroxide h Curd. C 19 H 29 COONa.
It is usually odourless but when heated to decomposition it smells like vinegar or acetic acid. However If you used dilute hydrochloric or sulphuric acid phosphoric acid and neutralize with. Calcium hydroxide b Oranges.
Sodium sulphate and water are formed when sulphuric acid reacts with sodium hydroxide a base. It is also used to make ammonium sulfate which is a particularly important fertilizer in sulfur-deficient. Sulfuric acid H 2 SO 4 is a strong acid with hygroscopic and oxidizing properties.
Sodium oxide combines with water to form sodium hydroxide. It has a strong acidic nature and is corrosive. Sodium AcetateCH3COONa- Sodium acetate is the salt of acetic acid and sodium hydroxide.
Acetone 20 o C. It is hygroscopic in nature and easily soluble in water. By far the largest amount of sulfuric acid is used to make phosphoric acid used in turn to make the phosphate fertilizers calcium dihydrogenphosphate and the ammonium phosphates.
Notice that you get the sodium salt formed rather than the carboxylic acid itself. Hydrochloric acid and strontium hydroxide. Sulphuric acid of course has all the reactions of a strong acid that you are familiar with from introductory chemistry courses.
Sodium Hydroxide NaOH Everyday we take a bath but water all alone is not enough to make us feel. Na 2 SO 4 10H 2 O. To learn more about Sodium Acetate Preparation Properties Uses and FAQs Visit.
Oxalic acid g Milk of magnesia. It is a strong acid. In this reaction methyl salicylate is reacted with sodium hydroxide to lead to the formation of a sodium salt intermediate of salicylic acid named disodium salicylate which upon undergoing further reaction with sulphuric acid leads to the formation of salicylic acid.
Citric Acid Anhydrous. Car batteries are usually lead batteries that contain sulphuric acidSulphuric acid can cause. It can be used for the preparation of salicylic acid.
Sodium hydroxide f Window cleaner. Sulphuric acid dissociates completely in an aqueous solution and produces H 2 and SO 4 2-. Chlorine Tablets 200g.
Sulfuric Acid is a mineral acid with the chemical formula H 2 SO 4. Sodium Hydroxide Micro-Pearls. Sodium Hydroxide is our main component in this titration process against oxalic acid.
Students acquire skills to perform a combination reaction. ChloricI acid reacts with sodium hydroxide. The most common application is the Sulphuric acid found in the car batteries.
And then hydrolysing methyl propanoate in the same way. Figure 1 Uses of sulfuric acid. 27 0 11 867 1184.
Formic acid c Vinegar. Formamidine Sulphuric Acid or Thiourea Dioxide. This mixture is relatively easy to.
Strontium hydroxide is a base. CH 4 N 2 SO 2. Sulfuric acid is also known as Mattling acid or Oil of vitriol.
Acetic acid i Ants sting. Acetamide 150 o C. Similar situation is with citric acid.
Makeup chemical in. As NaOH is less expensive it is more commonly used in products and manufacturing than KOH. Sulphuric acid is a strong acid whose single drop can cause a hollow mark in your skin.
Ammonium hydroxide conc 20 o C. Chemical Reagent High Purity Reagent Food Additives manufacturer supplier in China offering Used in Smelting Metal Welding Leather and Dye 995Min Boric Acid Flakes Lmprove Fastness of The Ceramics CAS 10043- 35-3995Min Boric Acid Flakes National Defense and Scientific Research Departments CAS 10043 -35-3 995Min Boric Acid Flakes and so on. First hydrolysing ethyl ethanoate using sodium hydroxide solution.
When an acid alkali neutralisation reaction happens - like when adding sodium hydroxide to sulfuric acid - it produces sodium sulfate and water. Apart from its too dangerous property it has numerous applications. Acetic acid 20 o C.
Lets understand some of the chemical properties of sodium hydroxide to understand the components used in the titration. At higher concentrations it acts as an oxidizing agent and dehydrating agent. Sodium Tripolyphosphate Food Grade Sodium Thiosulfate Pentahydrate.
Sulphuric Acid 20 Solution. Uses of sulfuric acid. Sodium hypochlorite Sulphuric acid Bac 50 Activated carbon Chlorine Sodium sulphite.
Sodium hydroxide NaOH also known as lye or caustic soda and potassium hydroxide KOH are the most common chemical agents. Ammonium Hydroxide Acetic Acid Potassium Chloride Hydrogen Peroxide Potassium Carbonate Formaldehyde. Citric acid d Lime water.
For example the normal reaction with sodium hydroxide solution is to form sodium sulphate solution - in which both of the acidic hydrogens react with hydroxide ions. Magnesium hydroxide j Spinach. In addition to removing stains in clothes sodium hypochlorite is also used to clean other types of stains such as mold and tea stains.
When nitric acid reacts with sodium hydroxide sodium nitrate and water are formed. Sodium Hexametaphosphate Tech Grade Sodium Carboxymethyl Cellulose Food Grade. Typically these solutions contain about 3-8 sodium hypochlorite and 001-005 sodium hydroxide added to reduce decomposition into sodium chlorate and sodium chloride.
HNO 3 NaOH NaNO 3 H 2 O. The salt strontium sulfate is formed with water as a product of the reaction between sulphuric acid and strontium hydroxide. The name of the salt is made by taking the first.
Formation of Sulphuric acid. Tartaric acid a Soap. Combination of sodium oxide and water.
Formation of sulphuric acid from sulphur trioxide is also a combination reaction. For causticizing of green liquor. Taking the same esters as above but using sodium hydroxide solution rather than a dilute acid.
It is widely used across a number of industrial sectors. H 2 SO 4 2NaOH Na 2 SO 4 2H 2 O. Sodium hydroxide is an odorless and white crystalline substance that absorbs moisture from the air at the environmental or surrounding.
Styrene Monomer Propylene Glycol Mono Ethylene Glycol. Lactic acid e Tamarind. Sulphuric Acid 10 Solution.

Sodium Hydroxide Sulfuric Acid Acid Base Neutralization Reaction Youtube
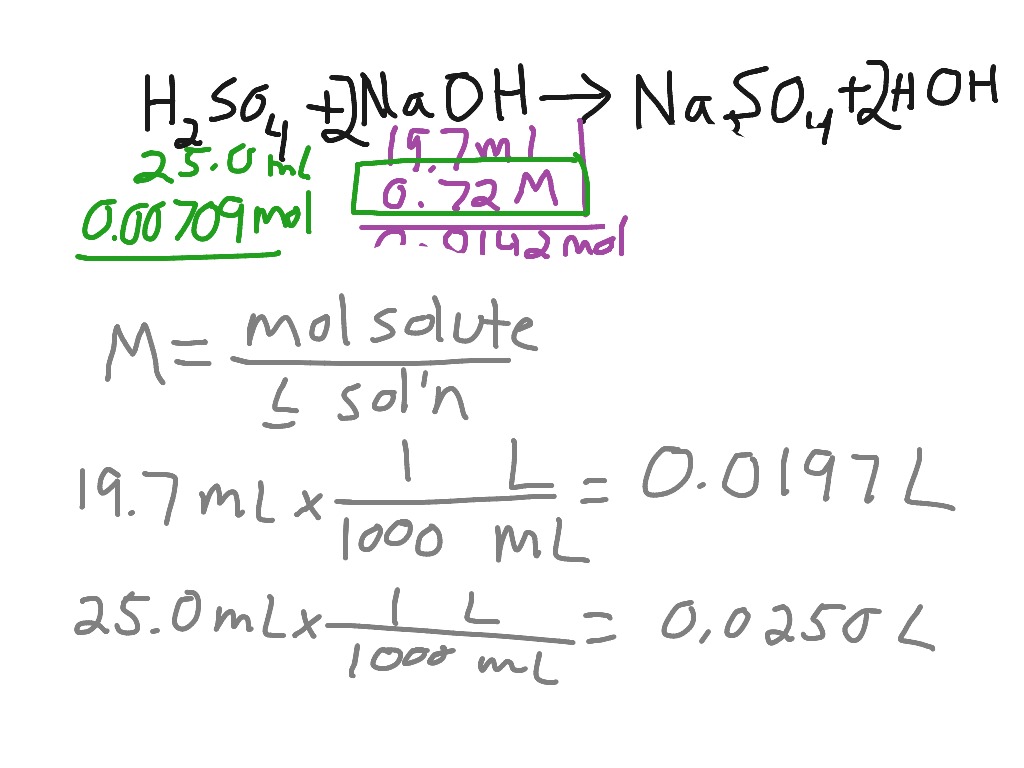
Titration Of Sulfuric Acid With Sodium Hydroxide Chemistry Acids And Bases Stoichiometry Showme

Sodium Hydroxide And Sulfuric Acid Yields Sodium Sulfate And Water Youtube
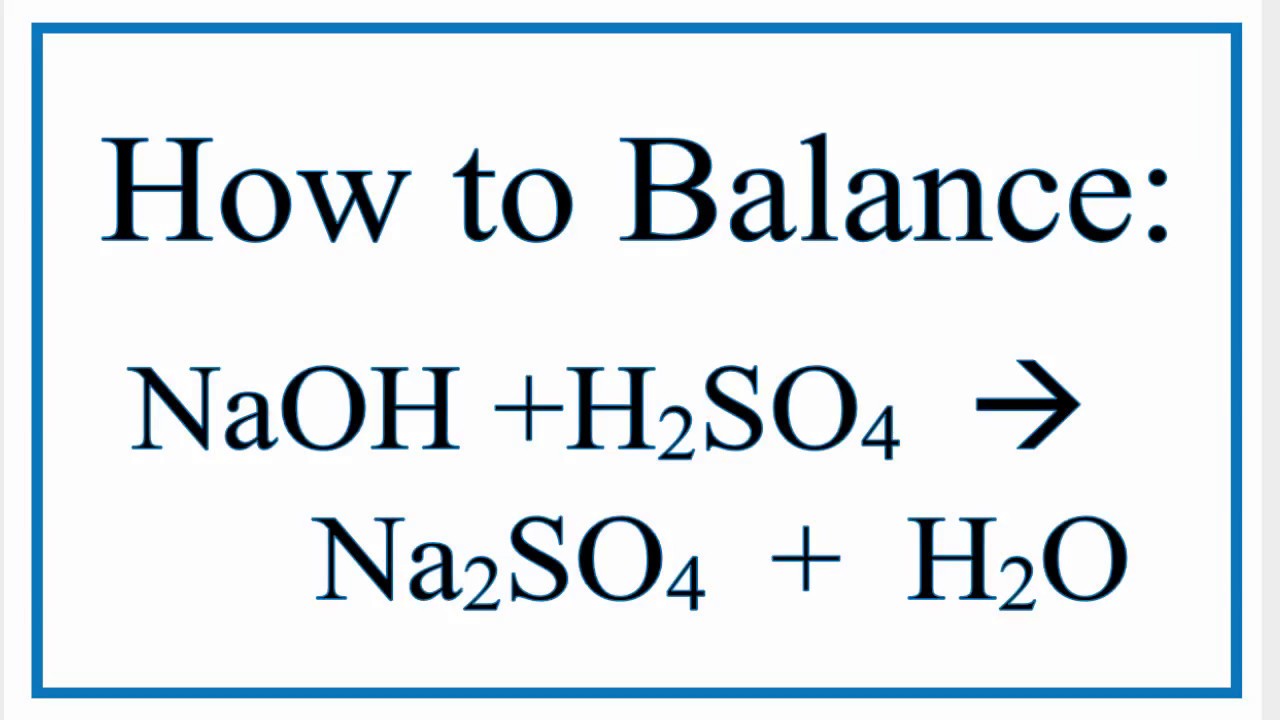
How To Balance Naoh H2so4 Na2so4 H2o Youtube
0 Response to "Sulphuric Acid and Sodium Hydroxide"
Post a Comment